ElendiLabs
在香港 MDACS 体系下,“制造商”是指对器械的设计、制造、包装和标签负最终法律责任的实体。如果您的香港总部掌握设计控制权且是 ISO 13485 证书的持有者,您可以尝试申请为“本地制造商”。 • 本土优势: 2026 年 CMPR 框架下,拥有“香港设计、香港监管”身份的产品在公立投标中通常拥有更高的技术评分。即使生产在内地,只要您的 QMS 体系将深圳工厂定义为受控的“外包供应商 (Outsourced Supplier)”,您仍有机会保留本地制造商的法律地位。
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 14, 2025
Approximately 5 minutes
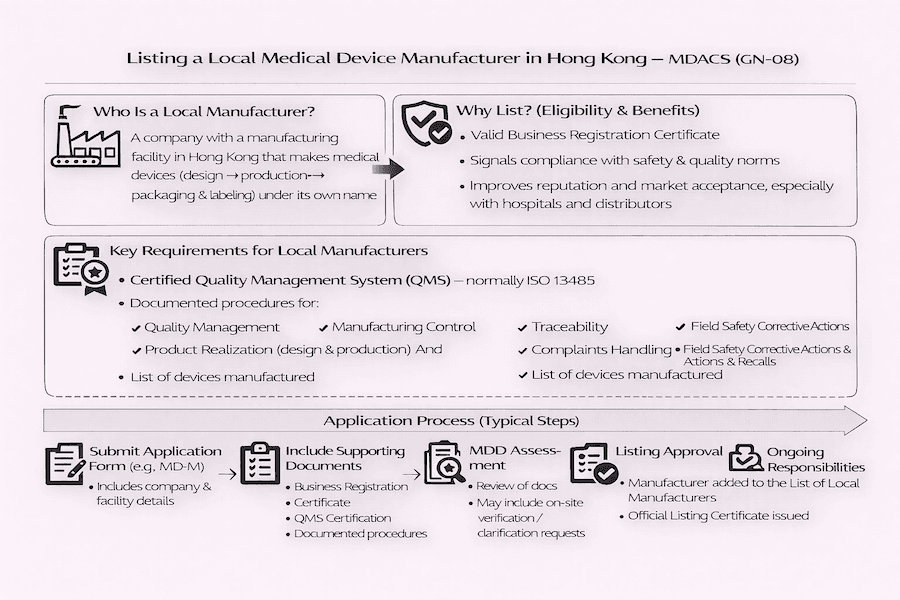
For manufacturers right here in Hong Kong who produce medical devices, the Medical Device Administrative Control System (MDACS) offers a voluntary listing scheme. What does this mean for local medical device manufacturing? It means there's a clear pathway to show your commitment to quality. The specific guidelines for local manufacturers are clearly laid out in the "Guidance Notes for Listing of Local Manufacturers of Medical Devices" (GN-08). This document, to our understanding, outlines the key criteria and steps for local manufacturing businesses to prove their dedication to producing safe and reliable medical devices in Hong Kong.
When we talk about "medical devices" in Hong Kong, what exactly does the Department of Health's Medical Device Division (MDD) consider to be one? According to their guidelines, a medical device is essentially any instrument, apparatus, machine, appliance, implant, software, material, or similar item. It's important that the manufacturer intends it to be used, either alone or with other things, for specific medical purposes in humans.
These purposes include:
Crucially, a medical device does not achieve its main effect in or on the body through drugs, immune responses, or metabolism. However, it might be helped in its function by such means. So, it's pretty broad, covering everything from a simple bandage to complex diagnostic software!
Under the MDACS, a "Local Manufacturer" refers to a company or entity that actively makes medical devices right here in Hong Kong. What does this manufacturing process typically involve? From our perspective, it includes everything from the initial design and production to the packaging and labeling of medical devices before they are officially put on the market under that manufacturer's own name. This definition helps clarify Hong Kong medical device manufacturing responsibilities.
Any legal company with a valid Business Registration Certificate and a manufacturing facility located in Hong Kong, producing medical devices covered by MDACS, can apply to be added to "The List of Local Manufacturers." While listing is voluntary, according to our experience, it offers some really significant benefits:
To get your Hong Kong medical device manufacturing operation listed under MDACS, you need to show you meet specific requirements, with a very strong focus on how you manage quality. What's the most important requirement?
The application process for getting your local medical device manufacturing operation listed typically involves these steps:
From our perspective, getting listed is a significant achievement, but it's not a one-time thing. Once you're on the list, local manufacturers have an ongoing duty to maintain their certified QMS, continuously follow their documented procedures, and promptly tell the MDD about any changes to their business information, manufacturing methods, or the medical devices themselves. Why is this commitment to continuous compliance so fundamental? Because it's what ensures the ongoing safety, quality, and effectiveness of medical devices made in Hong Kong, ultimately benefiting public health.
We'll follow up with you personally.
ElendiLabs
在香港 MDACS 体系下,“制造商”是指对器械的设计、制造、包装和标签负最终法律责任的实体。如果您的香港总部掌握设计控制权且是 ISO 13485 证书的持有者,您可以尝试申请为“本地制造商”。 • 本土优势: 2026 年 CMPR 框架下,拥有“香港设计、香港监管”身份的产品在公立投标中通常拥有更高的技术评分。即使生产在内地,只要您的 QMS 体系将深圳工厂定义为受控的“外包供应商 (Outsourced Supplier)”,您仍有机会保留本地制造商的法律地位。
Anonymous
目前 MDACS 的本地制造商表列主要是‘文件审核制’。但听说 2026 年底 CMPR 成立后会引入现场检查 (On-site Inspection)。对于我们这种租用小型无尘车间的本地企业,如果仓库空间不足或温控记录不全,会被直接撤销 HKMD 编号吗?LRP 在此类审计中承担什么法律责任?
ElendiLabs
是的,2026 年是香港监管从“被动接收”转向“主动飞行检查”的转折点。CMPR 审计将聚焦于追溯性 (Traceability) 和不良事件处理。 • 责任绑定: LRP 需要确保本地工厂的 SOP 符合 COP-01 守则。如果现场审计不合格,CMPR 有权吊销该制造商的所有相关产品列名。
Anonymous
我们是一家位于香港科学园的初创企业,生产一款 II 类物理治疗仪。目前我们的工厂表列申请仍处于‘补充资料’阶段。考虑到 2026 年 3 月 23 日 后,所有公立医院采购 (Stage C3) 必须持有最终的 HKMD 编号(不再接受 Stage B 的申请编号),如果我们在截止日期前未能拿到证书,是否意味着我们必须停供现有的医管局 (HA) 订单?有没有针对‘本地创新器械’的特殊加急通道?
ElendiLabs
2026 年 1 月的最新指引确认 Stage C 没有缓冲期。没有 HKMD 编号,产品将无法在 3 月 23 日后的标书中通过合规审查。 • 加急路径: 卫生署医疗器械分部 (MDD) 虽然没有官方的“加急费”政策,但对于在香港本地生产、且属于政府重点支持的创新器械(如入选 GBA 港澳药械通或科学园项目),LRP 可以向 MDD 提交 “采购紧急证明书 (Urgency Letter for Procurement)”,请求加速技术审评。ElendiLabs 建议您立即在 MDIS 门户 完善所有实验数据,以确保在最后 12 周内获批。
Approximately 5 minutes
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
This article details the application process for local medical device manufacturers seeking to be listed under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-08. It covers eligibility, quality management system requirements, and the submission process, all based on our insights and experience for effective Hong Kong medical device manufacturing.
Anonymous
我们的研发和质量控制总部在香港,但实际组装位于深圳的关联工厂。根据 GN-01 指南,我们是否可以申请‘本地制造商 (Local Manufacturer)’身份以获得香港政府采购的本土优先权?还是由于生产基地在内地,必须作为‘海外制造商’并通过‘表列进口商’模式进行注册?