ElendiLabs
具有“指定值 (Assigned Values)”的校准品和质控品必须与主试剂保持一致,即 Class D,不能降级。 • 附件区分: 只有不具备特定诊断功能的通用耗材(如普通的样本杯或非特异性洗涤液)可以作为 Class A。但在 2026 年的 MDIS 系统申报中,为了确保 Stage C 采购流程的完整性,建议将整个 Test System 作为一个“系统 (System)”进行整体列名,这样可以避免因配件表列号缺失而导致医院收货受阻。
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
June 2, 2025
Approximately 5 minutes
From our perspective, introducing In Vitro Diagnostic Medical Devices (IVDMDs) to the Hong Kong market requires careful navigation through the Medical Device Administrative Control System (MDACS). This system, run by the Department of Health, offers a clear framework for voluntarily listing medical devices. For those dealing with Class B, C, and D IVDMDs, the specific application procedures are well-explained in the "Guidance Notes for Listing Class B, C and D In Vitro Diagnostic Medical Devices" (GN-06). According to our experience, understanding this guidance is absolutely essential for manufacturers and Local Responsible Persons (LRPs) looking to bring these vital devices to Hong Kong.
Before you even start the listing process, it's super important to classify your IVDMD correctly. So, what exactly is an In Vitro Diagnostic Medical Device (IVDMD)? Simply put, these are medical devices used outside the human body to test samples (like blood or urine) to give you information for diagnosis, monitoring, or to check compatibility. To our understanding, similar to how general medical devices are classified, IVDMDs fall into four risk-based categories:
The classification of an IVDMD depends on factors like what it's intended to be used for, how experienced the person using it needs to be, how important the diagnostic information it gives is, and how the test results might affect an individual's health or public health. Technical Reference TR-006 gives detailed rules and examples for IVDMD classification. How are medical devices classified in Hong Kong generally? In Hong Kong, all medical devices are categorized based on their associated risk. This system helps ensure that devices with higher potential risks undergo more rigorous checks and controls to protect public safety. This classification is a foundational step in Hong Kong medical device registration.
Applications for adding Class B, C, or D IVDMDs to the MDACS List of Medical Devices must always be made by a Local Responsible Person (LRP). Who can actually be a Local Responsible Person (LRP)? From our experience, an LRP can be:
To our understanding, if you're a foreign manufacturer without a registered business in Hong Kong, you absolutely must appoint an LRP to act on your behalf. This is a critical step for IVDMD listing in Hong Kong.
The process for getting Class B, C, and D IVDMDs listed generally follows these steps, as laid out in GN-06:
Prepare Your Application Documents (Dossier):
Submit Online via MDIS:
MDD Assessment:
Listing and Certification:
By carefully following the procedures outlined in GN-06 and other relevant MDACS guidelines, manufacturers and LRPs can successfully navigate the IVDMD listing process in Hong Kong, ensuring these important devices are available for public health.
We'll follow up with you personally.
ElendiLabs
具有“指定值 (Assigned Values)”的校准品和质控品必须与主试剂保持一致,即 Class D,不能降级。 • 附件区分: 只有不具备特定诊断功能的通用耗材(如普通的样本杯或非特异性洗涤液)可以作为 Class A。但在 2026 年的 MDIS 系统申报中,为了确保 Stage C 采购流程的完整性,建议将整个 Test System 作为一个“系统 (System)”进行整体列名,这样可以避免因配件表列号缺失而导致医院收货受阻。
Approximately 5 minutes
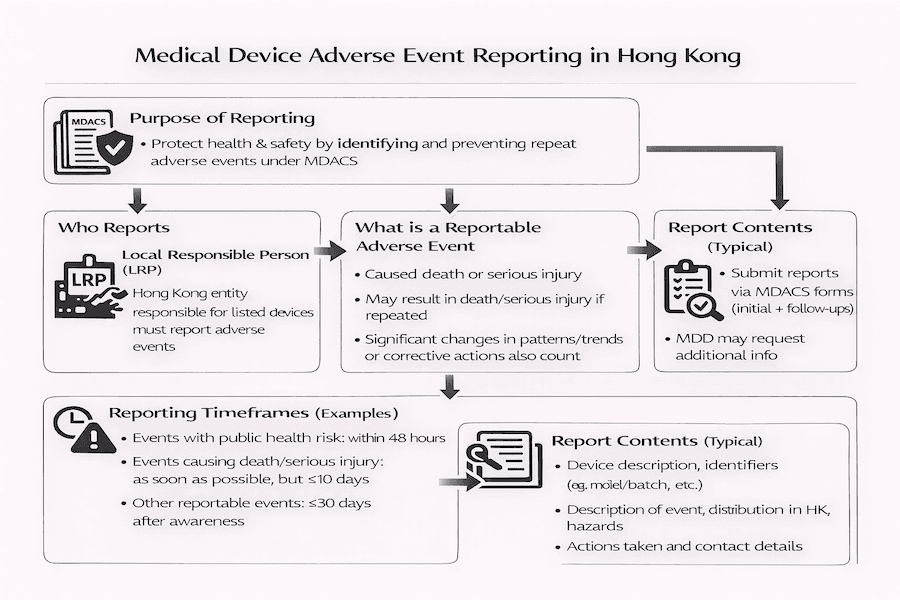
Adverse event reporting is a critical component of Hong Kong's Medical Device Administrative Control System (MDACS), aiming to enhance public health and safety. This article outlines the requirements and responsibilities of Local Responsible Persons (LRPs) in reporting adverse events related to listed medical devices, based on our insights.
Approximately 5 minutes
Complete guide to medical device regulations, classification, registration requirements and post-market surveillance in Hong Kong.
Approximately 5 minutes
Hong Kong's Medical Device Administrative Control System (MDACS) provides a robust, although currently voluntary, framework for regulating medical devices. This article explores the system's key features, including device classification, the listing process, the crucial role of Local Responsible Persons (LRPs), and its increasing importance for market access and public procurement, all from our insights and experience.
Approximately 5 minutes
This article details the application process for listing Class B, C, and D In Vitro Diagnostic Medical Devices (IVDMDs) under Hong Kong's Medical Device Administrative Control System (MDACS), as guided by GN-06. It covers classification, eligibility, submission requirements, and the online application via MDIS, all based on our insights and experience for efficient IVDMD listing in Hong Kong.
Anonymous
我们计划注册一套高风险传染病检测系统 (Class D)。系统中包含专用校准品、质控品以及通用的洗涤缓冲液。根据 TR-006 Rule 5 和 Rule 7,这些配件必须全部作为 Class D 申报吗?我们能否将通用的洗涤液作为 Class A 附件单独处理,以简化技术文档的更新