Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
March 20, 2024
Approximately 5 minutes
Registration of Pharmaceutical Products Containing a New Chemical or Biological Entity in Hong Kong
Registration of Pharmaceutical Products Containing a New Chemical or Biological Entity in Hong Kong
The registration of pharmaceutical products containing new chemical or biological entities (NCEs/NBEs) in Hong Kong follows a rigorous regulatory framework to ensure public health and safety. This guide provides comprehensive information about the registration process and requirements.
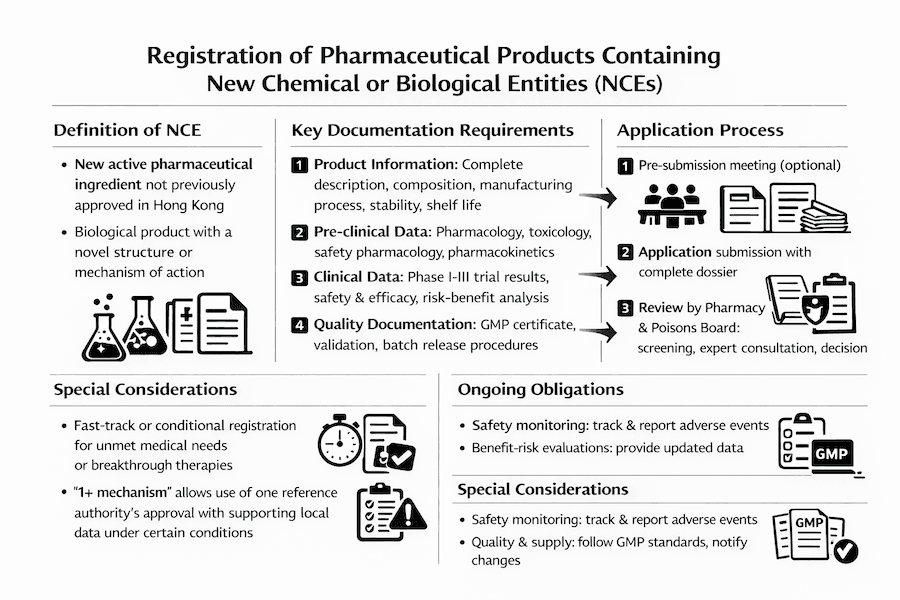
Definition of New Chemical or Biological Entity
A new chemical or biological entity is defined as:
- A new active pharmaceutical ingredient (API) that has not been previously approved in Hong Kong
- A substance that has not been previously registered as a pharmaceutical product
- A biological product with a new molecular structure or mechanism of action
Registration Requirements
Documentation Requirements
1. Product Information
- Complete product description
- Detailed composition
- Manufacturing process
- Quality control procedures
- Stability data
- Shelf life information
2. Pre-clinical Data
- Pharmacological studies
- Toxicological studies
- Safety pharmacology
- Pharmacokinetic data
3. Clinical Data
- Phase I, II, and III clinical trial results
- Safety and efficacy data
- Risk-benefit analysis
- Post-marketing surveillance plan
4. Quality Documentation
- Manufacturing license
- GMP compliance certificate
- Quality control specifications
- Validation protocols
- Batch release procedures
Application Process
-
Pre-submission Meeting
- Optional meeting with the Pharmacy and Poisons Board
- Discussion of application strategy
- Clarification of requirements
-
Application Submission
- Complete application form
- All required documentation
- Application fee payment
-
Review Process
- Initial screening
- Technical review
- Expert consultation
- Decision making
-
Post-approval Requirements
- Regular safety updates
- Periodic benefit-risk evaluation
- Post-marketing surveillance
- Adverse event reporting
Special Considerations
Fast Track Registration
Products may qualify for fast track registration if they:
- Address unmet medical needs
- Show significant therapeutic advantages
- Treat serious or life-threatening conditions
Conditional Registration
In certain cases, conditional registration may be granted with:
- Specific post-marketing commitments
- Additional safety monitoring requirements
- Limited initial approval period
Regulatory Compliance
Ongoing Obligations
- Regular safety updates
- Quality control monitoring
- Adverse event reporting
- Labeling updates
- Manufacturing changes notification
Post-marketing Surveillance
- Pharmacovigilance system
- Risk management plan
- Periodic safety update reports
- Signal detection and management
Best Practices
For Applicants
-
Early Planning
- Start preparation early
- Identify all requirements
- Plan for potential delays
-
Documentation
- Maintain complete records
- Ensure data integrity
- Follow formatting guidelines
-
Communication
- Regular updates to authorities
- Prompt response to queries
- Clear documentation
For Healthcare Professionals
-
Prescribing Considerations
- Review safety data
- Monitor patient response
- Report adverse events
-
Patient Education
- Explain benefits and risks
- Provide usage instructions
- Monitor compliance
Contact Information
For further information or assistance:
-
Pharmacy and Poisons Board
- Website: www.ppbhk.org.hk
-
Drug Office, Department of Health
- Website: www.drugoffice.gov.hk
Ask Anything
We'll follow up with you personally.