Back to Articles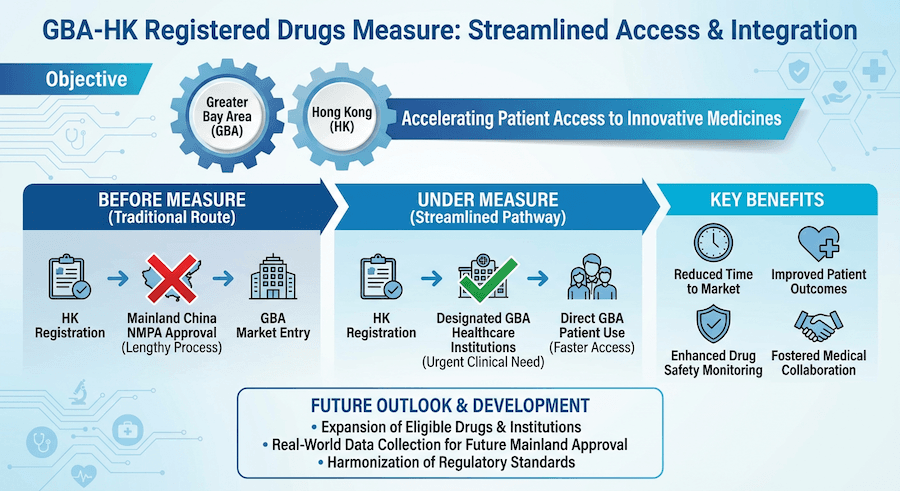
Need Regulatory Help? Try Our Platform
Post your regulatory questions or request quotations from verified pharmaceutical consultants worldwide. Get matched with experts who specialize in your market.
Registration
March 20, 2024
Approximately 5 minutes
Using Hong Kong Registered Drugs and Medical Devices in the Greater Bay Area: A Comprehensive Guide

Using Hong Kong Registered Drugs and Medical Devices in the Greater Bay Area: A Comprehensive Guide
The measure for using Hong Kong registered drugs and medical devices in the Guangdong-Hong Kong-Macao Greater Bay Area (GBA) represents a significant step forward in regional healthcare integration and accessibility.
Background and Purpose
Initiative Overview
- Launched in November 2020
- Part of the Work Plan for Regulatory Innovation and Development
- Aims to facilitate healthcare services for Hong Kong residents in the GBA
- Enables access to urgently needed clinical treatments
Key Objectives
- Improve healthcare accessibility
- Enhance cross-border medical services
- Facilitate regulatory innovation
- Support regional healthcare development
Implementation Progress
Timeline
- January 2021: Trial implementation at HKU-Shenzhen Hospital
- August 2021: Extension to nine GBA cities
- February 2023: Second batch of designated hospitals
- September 2024: Third batch of designated hospitals
Current Status
- 45 designated healthcare institutions
- 51 approved drugs
- 63 approved medical devices
- Coverage across major GBA cities
Designated Healthcare Institutions
First Batch (2021)
- University of Hong Kong-Shenzhen Hospital
- Modern Hospital Guangzhou
- Guangzhou United Family Hospital
- C-MER (Zhuhai) Dennis Lam Eye Hospital
- Zhongshan Chenxinghai Hospital
Second Batch (2023)
- Additional hospitals across GBA cities
- Expanded coverage of medical services
- Enhanced accessibility for patients
Third Batch (2024)
- Further expansion of healthcare network
- Increased treatment options
- Broader geographical coverage
Application Process
For Drugs
-
Eligibility Requirements
- Must be registered in Hong Kong
- Must have urgent clinical use
- Must meet safety standards
-
Application Steps
- Submit application to GDMPA
- Provide necessary documentation
- Await approval process
For Medical Devices
-
Eligibility Criteria
- Must be used in Hong Kong public hospitals
- Must have urgent clinical need
- Must meet safety requirements
-
Application Procedure
- Complete application forms
- Submit supporting documents
- Follow review process
Benefits and Impact
For Patients
-
Improved Access
- Faster availability of treatments
- Access to innovative therapies
- Enhanced healthcare options
-
Quality Care
- Standardized medical services
- Regulated product quality
- Professional healthcare support
For Healthcare System
-
Integration Benefits
- Cross-border healthcare coordination
- Resource optimization
- Enhanced service delivery
-
Development Opportunities
- Regional healthcare advancement
- Innovation promotion
- Quality improvement
Future Developments
Ongoing Expansion
- More designated hospitals
- Additional approved products
- Enhanced regulatory framework
Continuous Improvement
- Process optimization
- Policy refinement
- Service enhancement
Latest Updates
Recent Developments
- Regular updates to approved product lists
- Expansion of designated hospitals
- Enhancement of application procedures
Important Notices
- Check latest approved products
- Verify designated hospitals
- Follow updated guidelines
Ask Anything
We'll follow up with you personally.
100% response rate • Reply within 7 business days
Important Disclaimer